
January 25, 2024
Press Release
Measurement of lymphedema in the limbs using ZOZOSUITⓇ
――Achieving fast and simple limb measurements with high accuracy using ZOZOSUITⓇ――
<Announcement outline>
■It has been suggested that circumferential measurement of the limbs using the ZOZOSUIT®, a 3D body measurement suit and a lymphedema-specific smartphone app developed for validation may be useful as an assessment system for lymphedema.
■Circumferential limb measurements using this device offer an innovative, highly accurate method, providing quicker and easier measurements compared to traditional manual measurements.
■An application of this technology is expected to contribute to the practical use of a lymphedema assessment system which can be non-invasively repeated at home, without the need for a medical examiner.
*ZOZOSUIT is a registered trademark of ZOZO, Inc.
<Summary>
The Cancer Institute Hospital of JFCR (based in Koto City, Tokyo, Hospital Director: Takeshi SANO) Department of Plastic and Reconstructive Surgery, Ryo KARAKAWA, Tomoyuki YANO and ZOZO, Inc. (headquartered in Chiba City, Chiba Prefecture, Representative Director, President & CEO: Kotaro SAWADA) through joint research, investigated whether it is possible to measure the limb circumference of patients with secondary lymphedema after cancer surgery (ISL classification stages I ~ IIb) (*1), utilizing our 3D body measurement suit ZOZOSUIT®, along with a lymphedema-specific smartphone app developed for validation. The research findings suggest the potential usefulness of circumferential limb measurement using the device as an assessment system for lymphedema. This was presented by Karakawa et al. at the Annual Meeting of the American Society of Reconstructive Microsurgery (January 12 ~ 16, 2024).
Estimated to affect more than 150,000 individuals in Japan, lymphedema underscores the critical need for early detection and timely, targeted intervention to mitigate its progression. However, there is a disparity between the patient population and the availability of facilities which are capable of examining lymphedema.
This leads to patients with early or mild cases of lymphedema which warrant intervention, not receiving timely care.
The novel method using ZOZOSUIT® allows for fast, simple and accurate measurements, suggesting that it may be useful as an assessment system for lymphedema. An application of this technology is expected to lead to developing a new diagnosis tool which enables assessment of treatment effectiveness, early detection, and early intervention for lymphedema, repeatedly and non-invasively at home, without the need for medical supervision.
<Announcement>
(1)Background of research:
Lymphedema (Fig. 1), a condition characterized by swelling in the limbs due to lymph flow stagnation, particularly increases following surgeries for breast cancer and gynecological cancers in Japan. It is estimated that over 150,000 individuals nationwide are affected. This chronic, progressive condition leads to increased limb volume and weight, recurrent infections, and a decline in Quality of Life (QOL). Currently, there is no universally effective curative treatment. Therefore, a combination of conservative therapy, such as compression garments, and surgical interventions is necessary to prevent progression.
Early detection, and appropriate and timely intervention are crucial to preventing exacerbation. However, there is a shortage of facilities capable of examining lymphedema relative to the number of patients, resulting in insufficient intervention for patients with early or mild lymphedema cases who ideally should receive intervention.
To address these challenges, there is a need for the development of a simple and accurate assessment system for lymphedema. This system aims to prevent its progression in patients and streamline the delivery of care.
(2)Research content:
In this study, the research team conducted a joint research initiative with ZOZO, Inc., examining the feasibility of measuring the limb circumference of patients with secondary lymphedema after cancer resection (ISL classification stages I ~ IIb), utilizing our 3D body measurement suit ZOZOSUIT®, along with a lymphedema-specific smartphone app developed for validation. Subjects wearing the ZOZOSUIT® rotate 30° at a time, guided by the voice instructions via the app running on a smartphone placed 1.5m away. The twelve images captured are used to generate a 3D body surface model displayed in the app, and the circumferential measurements of important points for lymphedema treatment are extracted. This study validated the accuracy of repeated measurements using the system, comparing the measurement values and time with those obtained manually by conventional lymphedema treatment practitioners. The mean error for all measurement points was less than 10mm, and the mean absolute error compared to conventional methods was generally less than 20mm. Furthermore, the measurement time using this system was significantly shorter than manual measurements (86 seconds vs. 235 seconds, p<0.001). This suggests that the system, with its high accuracy and simple, fast measurement process, holds potential as an assessment system for lymphedema.
(3)Future prospects:
This research project is expected to lead to the development of a novel diagnostic tool. This tool will enable non-invasive, repeated assessments of treatment effectiveness, early detection, and early intervention for lymphedema at home without the need for a medical examiner. Furthermore, the accumulation of accurate data collected by this system holds the potential to contribute to building evidence for lymphedema treatment, addressing numerous unresolved challenges in the field. While there are still various challenges to overcome before this method can be practically implemented, its successful deployment could offer significant advantages. These include the early detection of subclinical lymphedema before it progresses to severe stages, promptly directing affected patients to appropriate medical facilities, and the possibility of remote lymphedema treatment. Reducing the number of cases that require major surgical intervention would benefit patients and is expected to lead to medical economic benefits.
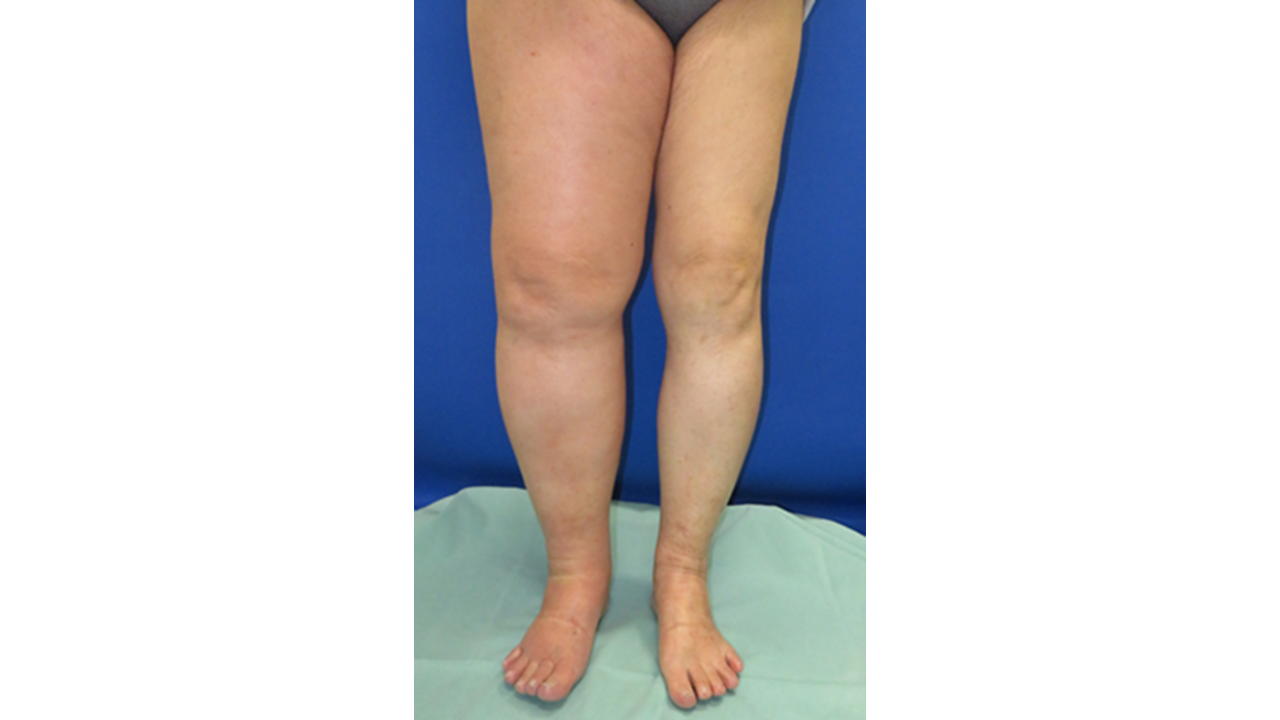
Fig.1: Image of a case of lymphedema
<Presenters>
This research was conducted as a joint research initiative between The Cancer Institute Hospital ofJFCR and ZOZO, Inc.
Title of Research Project: A Novel Lymphedema Monitoring Device Using Bodysuit and Smartphone: A Device Development Study
Principal Investigator (Organisation): Ryo KARAKAWA (Medical Director at The Cancer Institute Hospital of JFCR, Department of Plastic and Reconstructive Surgery)
Research team:
The Cancer Institute Hospital of JFCR
Department of Plastic and Reconstructive Surgery
Tomoyuki YANO (Chief)
Hidehiko YOSHIMATSU (Deputy chief)
Support Center for Patient and Family
Maiko OGASAWARA (Therapist)
Kuniko UTSUGI(Chief)
ZOZO, Inc.
Kawabata Lymphedema Clinic (Kobe)
<Glossary>
(*1) ISL classification stages I ~ IIb: Classification by the International Society of Lymphology (ISL)
Stage 0: A subclinical state where swelling is not evident despite impaired lymph transport. This stage may exist for months or years before edema becomes evident.
Stage I: This represents early onset of the condition where there is accumulation of tissue fluid that subsides with limb elevation. The edema may be pitting at this stage.
Stage IIa: Limb elevation alone rarely reduces swelling and pitting is manifest.
Stage IIb: There may or may not be pitting as tissue fibrosis is more evident.
Stage III: The tissue is hard (fibrotic) and pitting is absent. Skin changes such as thickening are seen.
ZOZO, Inc.
Contact PR from here